College of Medicine and Public Health
At the College of Medicine and Public Health, we deliver clinical, population and lab based research, integrated teaching programs and high quality clinical services.
It’s about improving the health and wellbeing for everyone in society.
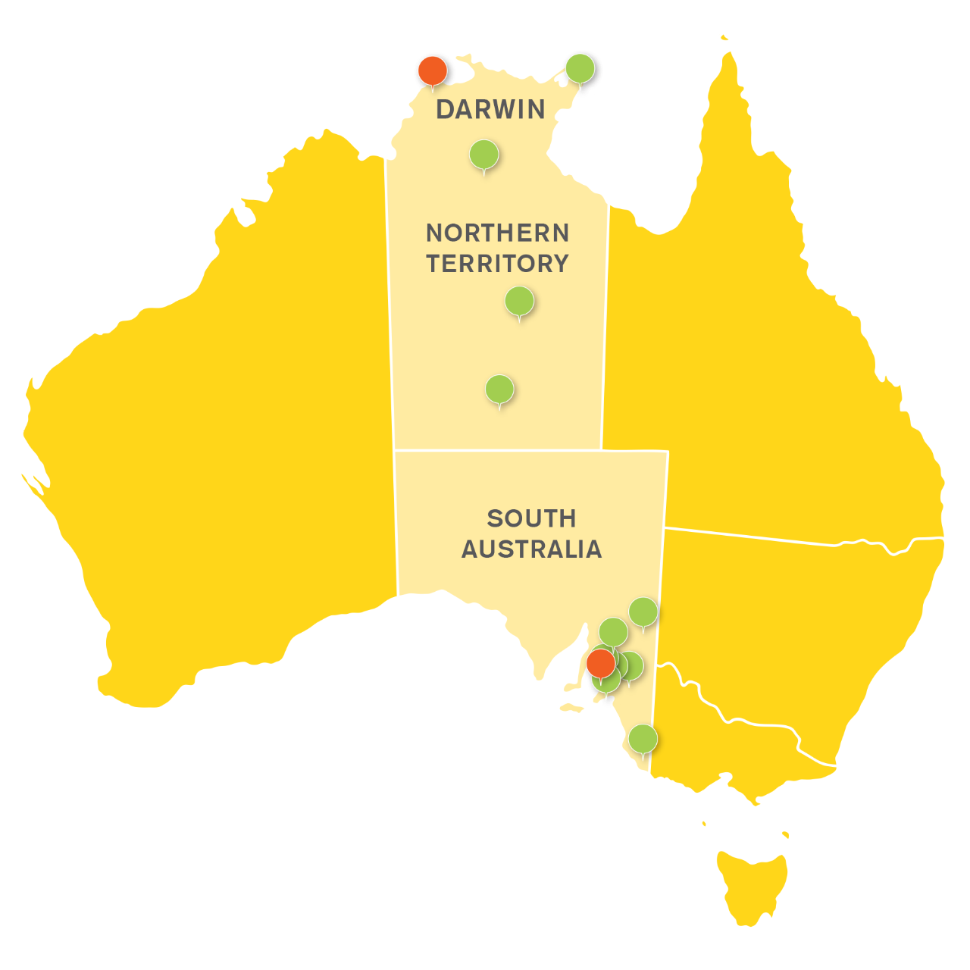
Our footprint reaches out from our world class teaching hospital at the Flinders Medical Centre in South Australia to multiple rural clinical locations all the way to Darwin in the Northern Territory.
We promote research in health services, systems improvements, public and population health, improved clinical care and laboratory and precision medicine.
This approach has seen us investigate everything from community health problems to the smallest of molecules that influence human disease.
Our research and teaching equips the next generation of leaders and innovators with the skill, commitment and vision to protect vulnerable communities and truly advance health outcomes. It’s at the heart of everything we do.
Rural and Remote Health SA & NT
Flinders University has a geographic footprint throughout the Central Australian Corridor.
The College of Medicine and Public Health’s collective vision is to be recognised as the global leader in producing a committed, highly skilled and culturally safe rural and remote health workforce, and rural and remote health research; both of which pay attention to the needs of our First Nations communities.

Scholarships
The College of Medicine and Public Health have a variety of scholarships available to students to help with their education.
Eligibility and scholarship criteria apply, please see within each scholarship opportunity listed for more information.
.jpg/_jcr_content/renditions/970.jpg)
Charting a new course through collaboration
The Poche SA + NT centre is focused on building healthy country, healthy communities and health leaders throughout Australia’s central corridor, across the Northern Territory and South Australia.
![]()
Sturt Rd, Bedford Park
South Australia 5042
South Australia | Northern Territory
Global | Online
CRICOS Provider: 00114A TEQSA Provider ID: PRV12097 TEQSA category: Australian University








